Change to Naseptin® Nasal Cream Formulation
Naseptin® Nasal Cream, containing the active ingredients chlorhexidine dihydrochloride and neomycin sulfate, is indicated for treatment of nasal infection with Staphylococci, and eradication of Staphylococci carriage from the nasal passages.
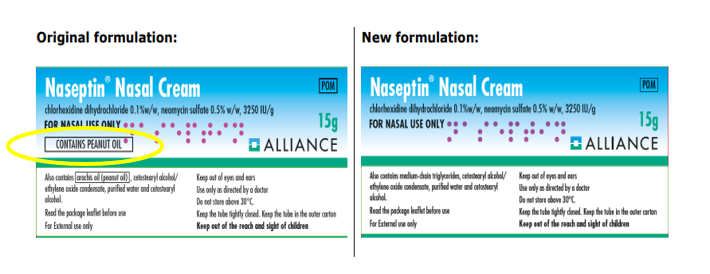
The original formulation contains the excipient arachis oil (peanut oil), which can induce an allergic response in some patients. As there is a possible relationship between allergy to peanut and allergy to soya, patients with soya allergy should also avoid the original Naseptin® formulation.
The cream has undergone a formulation change and arachis oil has been removed and replaced with medium chain triglycerides, to minimise this risk of allergic reactions.
The new arachis oil-free formulation has been in the supply chain since the end of April 2023. The last batch of the original formulation will expire on 31st October 2025. During the transition period (until November 2025), both formulations may be supplied when ordered.
The labelling on the original formulation contains a peanut oil boxed warning, the new formulation does not, see images of the cartons provided below:

Advice for Community Pharmacy teams
- When dispensing a prescription for Naseptin® Nasal Cream verify whether the patient has an allergy to peanut or to soya and if so, take care to dispense only the arachis oil-free formulation.
- Continue to report suspected adverse drug reactions (ADRs) to the MHRA through the Yellow Card Scheme.
Manufacturer’s contact details
Alliance Pharmaceuticals Limited - 01249 466 966 or medinfo@alliancepharma.co.uk